Abstract
Introduction
Historically, warfarin (Coumadin) was the only choice of anticoagulation in patients with chronic renal disease (CKD) stage V and end stage renal disease on dialysis (ESRD). Although apixaban (Eliquis) is approved for use in patients with CKD for venous thrombosis (VTE), its role in patients with CKD 5 and on dialysis is less defined. The purpose of this retrospective study is to compare the rates of bleeding, particularly gastrointestinal (GI) and intracranial bleeding in patients with CKD stage V or dialysis who are anticoagulated with warfarin or apixaban for indication of VTE.
Methods
Data for this study was queried from a commercial database (Explorys Inc, Cleveland, OH, USA), an aggregate of electronic health record data from 26 major integrated US healthcare systems representing a sixth of the US population. Cases and controls were identified using Systematized Nomenclature of Medicine (SNOMED) clinical terms or codes. Cases were defined as patients with CKD stage 5 or ESRD and were on apixaban for VTE. Controls were defined as those with CKD stage 5 or ESRD and were on warfarin for VTE. For the primary end point of bleeding, only patients above the age of 15 were selected. Those with previous history of GI or intracranial bleeding, atrial fibrillation, traumatic injury, cancer and cirrhosis were excluded. In the coumadin group, in addition to above, patients who were on for indication of artificial valves, arterial thrombosis and PVD were also excluded. 30 and 90-day rates of GI and intracranial bleeding were recorded for both groups. Logistic regression models were used to adjust of confounding variables (defined a priori as age > 65 or< 65, use of antiplatelet agents, gender, race and presence or absence of hypertension greater than 160/100 mm of hg). Rates or proportions were compared using Chi-squared test using Medcalc software (2018).Logistic regression analysis was done using Statistical Package for Social Sciences (SPSS, version 21, IBM Corp, Armonk, NY). P< 0.05 was considered statistically significant.
Results
A total of 990 cases and 8110 controls were identified. 30 and 90-day pooled GI and intracranial bleeding rates for apixaban users were 40% and 35% respectively. 30 and 90 day pooled GI and intracranial bleeding rates for warfarin users were 58% and 54% respectively. Difference in 30 day bleeding between cases and controls was 18% (95% CI 14.7-21.2; p< 0.0001) and 90 day bleeding was 19% (95% CI 15.7-22.1; p <0.001)
Due to the limitations of the study, both major and minor GI bleeds were included. Given this study was retrospective, efficacy data in preventing recurrent VTE could not be assessed.
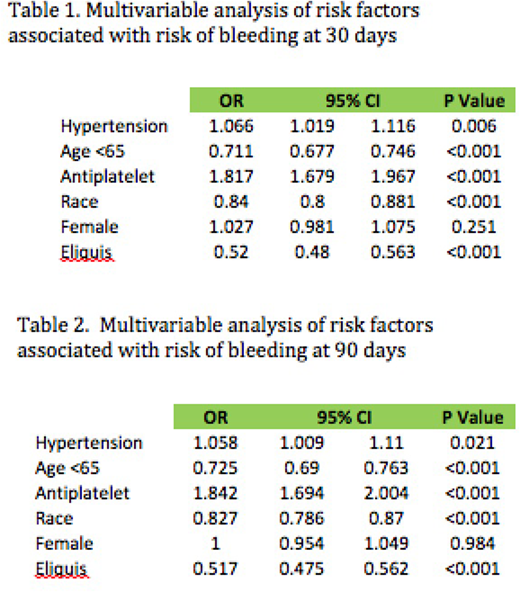
Logistic regression of risk factors associated with risk of bleeding revealed that age over 65 years, presence hypertension> 160/100 mm of Hg, use of antiplatelet agents, African american race, and use of warfarin as opposed to apixaban were all significantly associated with increased risk of both 30 and 90 day bleeding. Please see Table 1 and 2 for further details.
Discussion
Our study has shown that pooled rates of GI and intracranial bleeds were lower in the apixaban users even when used in patients with CKD stage V or ESRD patients, showing that apixaban is safe even when used for the indication of VTE prevention in this cohort. The results of this study are in concert with a recently published meta-analysis, which showed similar lower risks of bleeding when used in the advanced CKD cohort when used for the indication of atrial fibrillation. However, more studies are needed to prove its efficacy in this cohort.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal